Background
Parkinson disease (Parkinson's disease, PD) is a progressive neurodegenerative disorder associated with a loss of dopaminergic nigrostriatal neurons. It is named after James Parkinson, the English physician who described the shaking palsy in 1817.
Pathophysiology
The major neuropathologic findings in Parkinson disease are a loss of pigmented dopaminergic neurons in the substantia nigra and the presence of Lewy bodies. The loss of dopaminergic neurons occurs most prominently in the ventral lateral substantia nigra. Approximately 60-80% of dopaminergic neurons are lost before the motor signs of Parkinson disease emerge.
Lewy bodies are concentric, eosinophilic, cytoplasmic inclusions with peripheral halos and dense cores. The presence of Lewy bodies within pigmented neurons of the substantia nigra is characteristic, but not pathognomonic, of idiopathic Parkinson disease. Lewy bodies also are found in the cortex, nucleus basalis, locus ceruleus, intermediolateral column of the spinal cord, and other areas. Lewy bodies are not specific to Parkinson disease, as they are found in some cases of atypical parkinsonism, Hallervorden-Spatz disease, and other disorders. Incidental Lewy bodies are found at postmortem in patients without clinical signs of parkinsonism. The prevalence of incidental Lewy bodies increases with age. Incidental Lewy bodies have been hypothesized to represent the presymptomatic phase of Parkinson disease.
No standard criteria exist for the neuropathologic diagnosis of Parkinson disease, as the specificity and sensitivity of the characteristic findings have not been established clearly. Individuals presenting with primary dementia may exhibit neuropathologic features indistinguishable from those of Parkinson disease.
Alpha-synuclein is a major structural component of Lewy bodies. All Lewy bodies stain for alpha-synuclein and most also stain for ubiquitin.
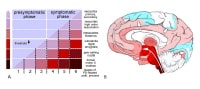
Stages in the development of Parkinson disease-related pathology. Adapted from Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Cell Tissue Res. 2004 Oct;318(1):121-34.
Recent studies demonstrate that Lewy-body pathology in Parkinson disease actually begins in the olfactory bulb and lower brainstem (see image above or Media file 4).1 These early stages are associated with premotor symptoms such as loss of sense of smell and rapid eye movement (REM) sleep behavior disorder (RBD).2 The pathology ascends up the brainstem to later involve the midbrain and nigrostriatal dopaminergic neurons. This stage correlates with onset of the motor phase of the disease and patients may exhibit bradykinesia, rigidity, and tremor. The pathology continues to ascend late in the disease to affect the cortex and patients may then exhibit cognitive dysfunction and dementia.
Motor circuit in Parkinson disease
The basal ganglia motor circuit modulates cortical output necessary for normal movement (see following image or Media file 1).
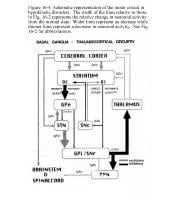
Schematic representation of the basal ganglia - thalamocortical motor circuit and its neurotransmitters in the normal state. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson's disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:240. Used with kind permission. Copyright, McGraw-Hill Companies, Inc.
Signals from the cerebral cortex are processed through the basal ganglia-thalamocortical motor circuit and return to the same area via a feedback pathway. Output from the motor circuit is directed through the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). This inhibitory output is directed to the thalamocortical pathway and suppresses movement.
Two pathways exist within the basal ganglia circuit; they are referred to as the direct and indirect pathways. In the direct pathway, outflow from the striatum directly inhibits GPi and SNr. The indirect pathway comprises inhibitory connections between the striatum and the external segment of the globus pallidus (GPe) and the GPe and the subthalamic nucleus (STN). The subthalamic nucleus exerts an excitatory influence on the GPi and SNr. The GPi/SNr sends inhibitory output to the ventral lateral (VL) nucleus of the thalamus. Striatal neurons containing D1 receptors constitute the direct pathway and project to the GPi/SNr. Striatal neurons containing D2 receptors are part of the indirect pathway and project to the GPe.
Dopamine is released from nigrostriatal (SNc) neurons to activate the direct pathway and inhibit the indirect pathway. In Parkinson disease, decreased striatal dopamine causes increased inhibitory output from the GPi/SNr (see following image or Media file 2).
Schematic representation of the basal ganglia - thalamocortical motor circuit and the relative change in neuronal activity in Parkinson disease. From Vitek J. Stereotaxic surgery and deep brain stimulation for Parkinson's disease and movement disorders. In: Watts RL, Koller WC, eds. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill, 1997:241. Used with kind permission. Copyright, McGraw-Hill Companies, Inc.
This increased inhibition of the thalamocortical pathway suppresses movement. Via the direct pathway, decreased striatal dopamine stimulation causes decreased inhibition of the GPi/SNr. Via the indirect pathway, decreased dopamine inhibition causes increased inhibition of the GPe, resulting in disinhibition of the STN. Increased STN output increases GPi/SNr inhibitory output to the thalamus.
Frequency
International
The incidence has been estimated to be 4.5-21 cases per 100,000 population per year. Estimates of Parkinson disease prevalence range from 18-328 per 100,000 population, with most studies yielding a prevalence of approximately 120 per 100,000.
Sex
Parkinson disease is about 1.5 times more common in men than in women.
Age
The incidence and prevalence of Parkinson disease increase with age. The average age of onset is approximately 60 years. Onset in persons younger than 40 years is relatively uncommon.
Clinical
">
History
- Parkinson disease may have a long premotor phase. Mid-life risk factors for the later development of Parkinson disease include constipation and daytime sleepiness. These may well be the first clinical manifestations of the disease but are nonspecific. Additional features that commonly precede onset of motor signs include decreased sense of smell and REM behavior disorder (RBD).
- REM behavior disorder is a sleep disorder in which there is a loss of normal atonia during REM sleep.
- Patients are observed by their bed partners to “act out their dreams” and the partners may note kicking, hitting, talking, or crying out.
- In one study, 38% of 50-year-old men with REM behavior disorder and no neurologic signs went on to develop Parkinsonism.3
- REM behavior disorder is common throughout the course of Parkinson disease.
- Onset of motor signs in Parkinson disease is typically asymmetric, with the most common initial finding being an asymmetric resting tremor in an upper extremity. About 20% of patients first experience clumsiness in one hand. Over time, patients notice symptoms related to progressive bradykinesia, rigidity, and gait difficulty.
- Tremor usually begins in one upper extremity and initially may be intermittent. As with most tremors, the amplitude increases with stress and resolves during sleep. After several months or years, the tremor may affect the extremities on the other side, but asymmetry is usually maintained. Parkinson disease tremor may also involve the lower extremities, tongue, lips, or chin.
- The initial symptoms of Parkinson disease may be nonspecific and include fatigue, depression, constipation, and sleep problems.
- Some patients experience a subtle decrease in dexterity and may notice a lack of coordination with activities such as playing golf or dressing.
- Some patients complain of aching or tightness in the calf or shoulder region.
- The first affected arm may not swing fully when walking, and the foot on the same side may scrape the floor.
- Over time, axial posture becomes progressively flexed and strides become shorter.
- Decreased swallowing may lead to excess saliva in the mouth and ultimately drooling.
- Symptoms of autonomic dysfunction are common and include constipation, sweating abnormalities, sexual dysfunction, and seborrheic dermatitis.
- Sleep disturbances are common.
- The best clinical predictors of a pathology diagnosis of Parkinson disease are the following:
- Asymmetry
- Presence of resting tremor
- Good response to dopamine replacement therapy
- Long-term disability in Parkinson disease is usually related to dementia and balance dysfunction.
Physical
The 3 cardinal signs of Parkinson disease are resting tremor, rigidity, and bradykinesia. Of these cardinal features, 2 of 3 are required to make the clinical diagnosis. Postural instability (balance dysfunction) is the fourth cardinal sign, but it emerges late in the disease, usually after 8 years or more.
- The characteristic Parkinson disease tremor is present and most prominent with the limb at rest.
- The usual frequency is 3-5 Hz.
- The tremor may appear as a pill-rolling motion of the hand or a simple oscillation of the hand or arm.
- The same tremor may be observed with the arms outstretched (position of postural maintenance) and a less prominent, higher frequency kinetic tremor is also common.
- Rigidity refers to an increase in resistance to passive movement about a joint.
- The resistance can be either smooth (lead pipe) or oscillating (cogwheeling).
- Cogwheeling is thought to reflect tremor rather than rigidity and may be present with tremors not associated with an increase in tone (ie, essential tremor).
- Rigidity usually is tested by flexing and extending the patient's relaxed wrist.
- Rigidity can be made more obvious with voluntary movement in the contralateral limb.
- Bradykinesia refers to slowness of movement but also includes a paucity of spontaneous movements and decreased amplitude of movement. Bradykinesia is also expressed as micrographia (small handwriting), hypomimia (decreased facial expression), decreased blink rate, and hypophonia (soft speech).
- Postural instability refers to imbalance and loss of righting reflexes. Its emergence is an important milestone, because it is poorly amenable to treatment and a common source of disability in late disease.
- Patients may experience freezing when starting to walk (start-hesitation), during turning, or while crossing a threshold, such as going through a doorway.
- Dementia generally occurs late in Parkinson disease and affects 15-30% of patients. Short-term memory and visuospatial function may be impaired, but aphasia is not present. Cognitive dysfunction within a year of onset of motor features suggests a diagnosis of Lewy body disease, a disease closely related to Parkinson disease and marked by the presence of cortical Lewy bodies. See Parkinson Disease Dementia for more information.
Causes
Most cases of idiopathic Parkinson disease are believed to be due to a combination of genetic and environmental factors. At both ends of the spectrum are rare cases that appear to be due solely to one or the other.
- Environmental risk factors associated with the development of Parkinson disease include use of pesticides, living in a rural environment, consumption of well water, exposure to herbicides, and proximity to industrial plants or quarries.
- Several individuals have been identified who developed parkinsonism after self-injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).4
- These patients developed bradykinesia, rigidity, and tremor, which progressed over several weeks and improved with dopamine replacement therapy.
- MPTP crosses the blood-brain barrier and is oxidized to MPP+ by the enzyme monoamine oxidase (MAO) type B.
- MPP+ accumulates in mitochondria and interferes with the function of complex I of the respiratory chain.
- A chemical resemblance between MPTP and some herbicides and pesticides suggested that an MPTP-like environmental toxin might be a cause of Parkinson disease, but no specific agent has been identified. Nonetheless, mitochondrial complex I activity is reduced in Parkinson disease, suggesting a common pathway with MPTP-induced parkinsonism.
- The oxidation hypothesis suggests that free radical damage, resulting from dopamine's oxidative metabolism, plays a role in the development or progression of Parkinson disease.
- The oxidative metabolism of dopamine by MAO leads to the formation of hydrogen peroxide. Hydrogen peroxide normally is cleared rapidly by glutathione.
- If hydrogen peroxide is not cleared adequately, it may lead to the formation of highly reactive hydroxyl radicals that can react with cell membrane lipids to cause lipid peroxidation and cell damage. In Parkinson disease, levels of reduced glutathione are decreased, suggesting a loss of protection against formation of free radicals. Iron is increased in the substantia nigra and may serve as a source of donor electrons, thereby promoting the formation of free radicals.
- Indices of lipid peroxidation are increased in Parkinson disease.
- Thus, Parkinson disease is associated with increased dopamine turnover, decreased protective mechanisms (glutathione), increased iron (a pro-oxidation molecule), and evidence of increased lipid peroxidation. This hypothesis raised concern that increased dopamine turnover due to levodopa administration could increase oxidative damage and accelerate loss of dopamine neurons. However, there is no clear evidence that levodopa accelerates disease progression.
- The role of genetic factors has been studied in twins.
- If genetic factors are important, concordance in genetically identical monozygotic (MZ) twins will be greater than in dizygotic (DZ) twins, who share only about 50% of genes. Early twin studies generally found low and similar concordance rates for MZ and DZ pairs.
- In a recent study of 193 twins, overall concordance for MZ and DZ pairs was similar. However, in 16 pairs of twins in whom Parkinson disease was diagnosed at or before age 50 years, all 4 MZ pairs, but only 2 of 12 DZ pairs, were concordant. This suggests that while genetic factors may not be very important when the disease begins after age 50 years, genetic factors appear to be very important when the disease begins at or before age 50 years.
- The identification of a few large families with apparent familial Parkinson disease sparked further interest in the genetics of the disease.
- One large family with highly penetrant, autosomal-dominant, autopsy-proven Parkinson disease originated in the town of Contursi in the Salerno province of southern Italy. Of 592 family members, 50 were affected by Parkinson disease. These individuals were characterized by early age of disease onset (mean age 47.5 y), rapid progression (mean age at death 56.1 y), lack of tremor, and good response to levodopa therapy.
- Linkage analysis incriminated a region in chromosome bands 4q21-23, and sequencing revealed an A-for-G substitution at base 209 of the alpha-synuclein gene. Termed PD-1, this mutation codes for a substitution of threonine for alanine at amino acid 53.
- Five small Greek kindreds also were found to have the PD-1 mutation. In a German family, a different point mutation in the alpha-synuclein gene (a substitution of C for G at base 88, producing a substitution of proline for alanine at amino acid 30) confirmed that mutations in the alpha-synuclein gene can cause Parkinson disease. A few additional familial mutations in the alpha-synuclein gene have been identified and are now collectively called PARK1. It is now clear that these mutations are an exceedingly rare cause of Parkinson disease.
- Alpha-synuclein is a major component of Lewy bodies in all Parkinson disease.
- All Lewy bodies stain for alpha-synuclein, and most also stain for ubiquitin, which conjugates with proteins targeted for proteolysis. Abnormal aggregation of alpha-synuclein into filamentous structures may precede ubiquitization.
- One hypothesis states that the PD-1 mutation alters the configuration of alpha-synuclein into a ß structure that could aggregate into sheets.
- All Parkinson disease may be associated with abnormal folding of alpha-synuclein, leading to excessive aggregation and neuronal death.
- Although sporadic Parkinson disease is not caused by a mutation in the alpha-synuclein gene, active investigation is underway into proteins that interact with alpha-synuclein, including those that guide, promote, or prevent aggregation of the protein.
- As Parkinson disease, dementia with Lewy bodies, and multiple system atrophy (MSA) all exhibit Lewy bodies that stain for alpha-synuclein, they have been designated "alpha-synucleinopathies."
- A recent hypothesis suggests that Parkinson disease is caused by abnormalities of the proteosome system, which is responsible for clearing abnormal proteins.
- Several homozygous deletions in a gene dubbed parkin (PARK2), which is located on chromosome 6, have been found to cause autosomal-recessive juvenile parkinsonism (AR-JP). This form of parkinsonism differs pathologically from Parkinson disease in that no Lewy bodies are found in the substantia nigra.
- Several other gene abnormalities have been identified in families with Parkinson disease and these are designated PARK3 -PARK12.
- It has been estimated that all currently known genetic causes of Parkinson disease account for less than 5% of Parkinson disease cases.


No comments:
Post a Comment